Services
RTSM
Revolutionary technology for your clinical trials
In 2022, by integrating the Randomisation and Trial Supply Management (RTSM) service, THERADIS strengthens its support for clinical projects with methodological expertise, logistical optimisation and real data integration
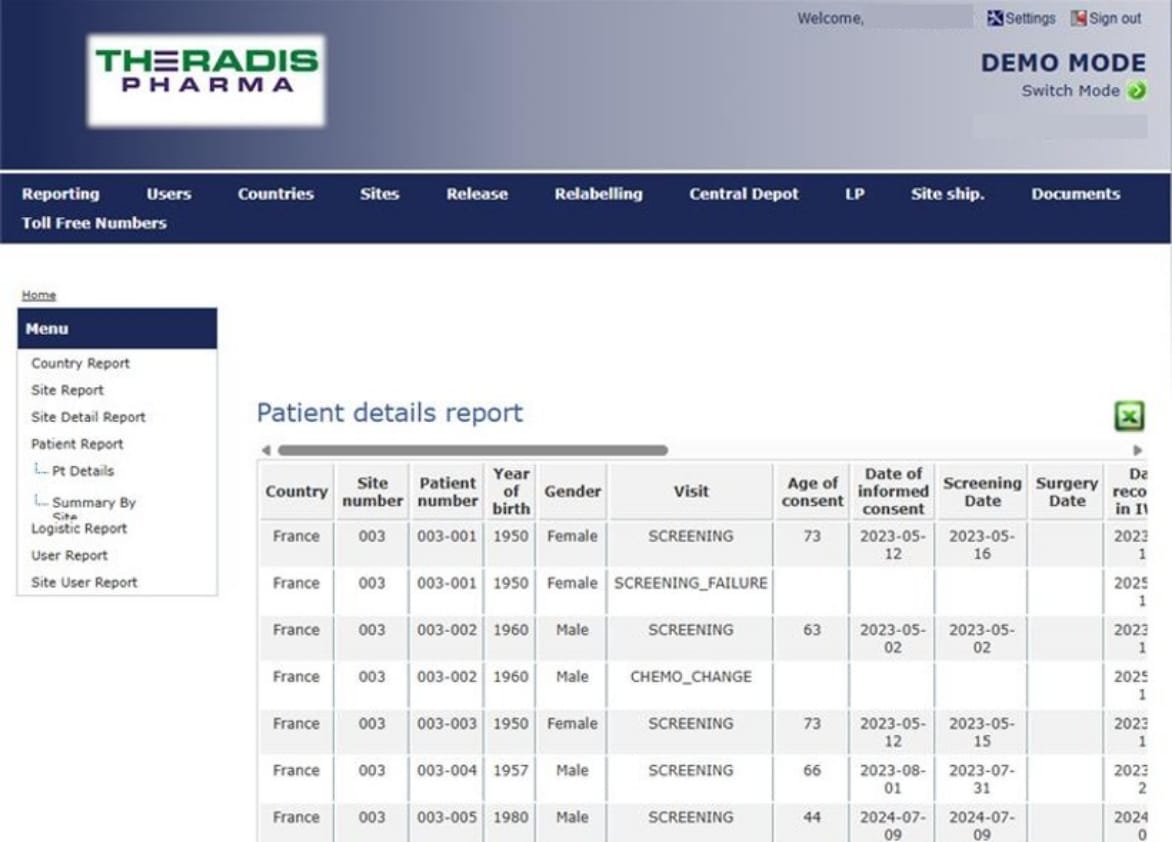
The RTSM system enables you to monitor the progress of your study in real time. Using an online platform, it assigns a treatment number to a randomised patient, tracks stock levels, and can provide alerts and live activity reports.
STATISTICAL AND METHODOLOGICAL SUPPORT
• Consultation during project preparation
• Complex randomisation simulation and validation
• In-house consultant to avoid
randomisation errors (partial unblind, etc.)
• Internal advisor to prepare the procedure in the event of a problem (treatment allocation without authorisation)
• Complex randomisation simulation and validation
• In-house consultant to avoid
randomisation errors (partial unblind, etc.)
• Internal advisor to prepare the procedure in the event of a problem (treatment allocation without authorisation)
PATIENTS AND INVESTIGATOR SITE MANAGEMENT
• Randomisation / Emergency code
in case of break-in (no sealed envelope on site)
• Recruitment control
• Complex treatment allocation model
(titration, replacement)
• Email and fax
confirmation notifications, system alerts
in case of break-in (no sealed envelope on site)
• Recruitment control
• Complex treatment allocation model
(titration, replacement)
• Email and fax
confirmation notifications, system alerts
LOGISTICS MANAGEMENT
• Product availability
• Cost optimisation according to :
Methodological constraints, Treatment availability,
Treatment stability, Cost of treatment,
Site storage capacity
• Clinical supplies management
• Clinical samples management
• Routine Treatment Units management: deactivation,
quarantine, expiry, relabelling, destruction
• Cost optimisation according to :
Methodological constraints, Treatment availability,
Treatment stability, Cost of treatment,
Site storage capacity
• Clinical supplies management
• Clinical samples management
• Routine Treatment Units management: deactivation,
quarantine, expiry, relabelling, destruction

AUTOMATED LINK
With other parties involved in the study to download or transfer data
• Central laboratories
• Imaging, ECG, ePro, etc.
• EDC
• Local depots, central pharmacies
• Any other external source
• Imaging, ECG, ePro, etc.
• EDC
• Local depots, central pharmacies
• Any other external source
STUDY FOLLOW-UP
• Real-time reporting
• Customisable to specific needs
• Continuous monitoring and alert system (number of patients selected/randomised;
availability/expiry of treatments)
24/7 ASSISTANCE
• Hotline support and live chat
• Immediate translation through an interpreter
• Immediate translation through an interpreter