Management of Investigational Medicinal Products
Clinical Trial Logistics
Clinical Trial Logistics
You are here : Accueil >
Process
The process
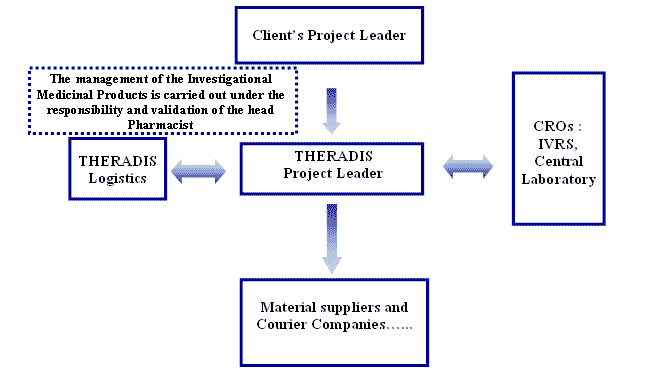
Logistics Communication Flow chart
Process
Technical meeting with the client
Drawing up and validation of Study Specifications
Staff training
Reception of study material or IMP
Recording reception
Identification and storage in study reserved zone
Assembly, manufacturing, programming,
packaging and labelling
Reception of supply request (fax, email, IVRS)font>
Shipment preparation
Transport organisation
Recording of shipment
Verification of reception of material by site
Resupply upon request
Recording of acknowledgement of receipt
Organisation of recuperation of material
Recording returns
Destruction of material or return to Sponsor on Project Leader’s request
Status tables available « on-line »
Follow up meetings with client