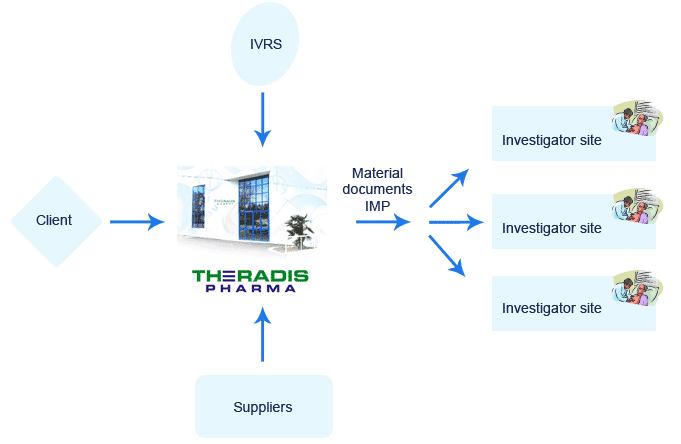
Consolidated Logistics
Consolidated logistics involves the use of a unique platform capable of assuming the entire logistic chain for the distribution of all types of material, documents, biological sampling kits and IMP required for a clinical trial.
The advantages :
- Establishment of an effective and clear communication flow as there is a unique « Distributor ».
- Reduced number of operators.
- A unique data base verified and validated, containing the contact details of investigation sites, thus limiting the risk of error.
- Optimisation of the number of IMP, CRFs and sampling kits as a reserve stock is not required.
- Centralised management of stock.
- Permanent access to status of stock, of material, documents and IMP.
- Reduction in number of shipments as material, documents, sampling kits and in certain cases IMP, are sent at the same time.
- Transport costs greatly optimised.
- Time saved in the preparation of shipments.
- Excellent perception by investigators who have a unique contact for all study shipments.
- Possibility of making the supply, resupply and return of material, automatic.